- Products
- 3D Printing Consumables
- 3D PRINTING RESINS - DLP AND MSLA FORMULAS
- DLP 3D Printer Resins (eg. Asiga etc)
- Detax Freeprint SPLINT 2.0 (HARD) 385 DLP 3D Printing Resin 02076 - 1000g
Detax Freeprint SPLINT 2.0 (HARD) 385 DLP 3D Printing Resin 02076 - 1000g
- Brand: Detax
- Product Code: D-FP-S-1000
3D Print Resin Stock Holding Information and Leadtimes:
Initally given the nature of the digital landscape in the dental industry, Durodent will only be stocking the most common requested products and colours.
Supplementary products and shades maybe added to our range and ordered on request (2 - 4 weeks leadtimes).
If you have particular interest in other ranges like Gingiva, Model, Cast, Thermoforming Heat Resistant Model resins etc please contact us to discuss.
Detax FREEPRINT® splint 2.0
* Please check IFU (Instruction For Use) for Validated Medical Device (MD) Process and Equipment
* Asiga and Otoflash Validated
* ARTG Registered 413802
Made In Germany - Detax is a leading trusted chemical and dental material manufacture
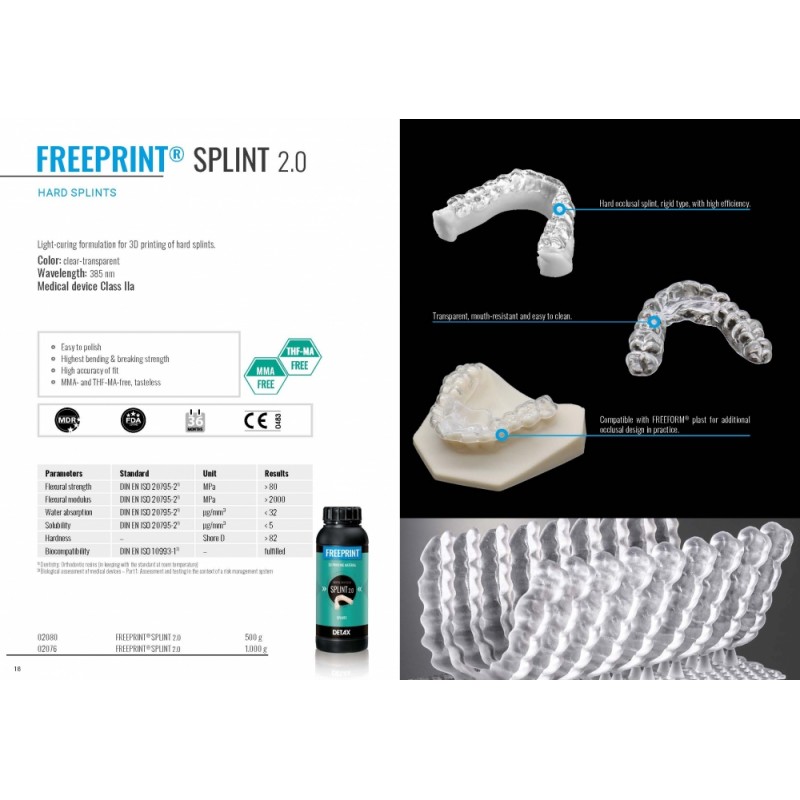
Light-curing, biocompatible resin
Light-curing formulation for 3D printing of hard splints.
Color: clear-transparent
Wavelength: 385 nm
Medical device Class IIa
-
Process Validation
-
385nm DLP Printers: Asiga (All), Microlay, Miicraft, Rapid Shape, Straumann, W2P
-
Curing Devices: Otoflash, Dentalfarm
-
-
Easy to polish
-
Highest bending & breaking strength
-
High accuracy of fit
-
MMA- and THF-MA-free, tasteless
Clear-transparent formulation for visual control in the working area. Process reliability through high initial hardness and final strength.
Low-viscosity approach for low material consumption and more rapid cleaning. Highest mechanical bending and breaking strength, without being brittle.
Neutral odour and taste.
TGA Registered Medical device Classs IIa
1) Dentistry: Orthodontic resins (in keeping with the standard at room temperature)
2) Biological assessment of medical devices – Part 1: Assessment and testing in the context of a risk management system
|
Parameters |
Standard |
Unit |
Results |
|
Flexural strength Flexural modulus |
DIN EN ISO 20795-21) DIN EN ISO 20795-21) |
MPa MPa |
> 80 > 2000 |
|
Water absorption Solubility Hardness |
DIN EN ISO 20795-21) DIN EN ISO 20795-21) – |
μg/mm³ μg/mm³ Shore D |
< 32 < 5 > 82 |
|
Biocompatibility |
DIN EN ISO 10993-12) |
– |
fulfilled |
Detax Freeprint SPLINT 2.0 (HARD) 385 DLP 3D Printing Resin 02076 - 1000g
- Brand: Detax
- Product Code: D-FP-S-1000
3D Print Resin Stock Holding Information and Leadtimes:
Initally given the nature of the digital landscape in the dental industry, Durodent will only be stocking the most common requested products and colours.
Supplementary products and shades maybe added to our range and ordered on request (2 - 4 weeks leadtimes).
If you have particular interest in other ranges like Gingiva, Model, Cast, Thermoforming Heat Resistant Model resins etc please contact us to discuss.
Detax FREEPRINT® splint 2.0
* Please check IFU (Instruction For Use) for Validated Medical Device (MD) Process and Equipment
* Asiga and Otoflash Validated
* ARTG Registered 413802
Made In Germany - Detax is a leading trusted chemical and dental material manufacture
Light-curing, biocompatible resin
Light-curing formulation for 3D printing of hard splints.
Color: clear-transparent
Wavelength: 385 nm
Medical device Class IIa
-
Process Validation
-
385nm DLP Printers: Asiga (All), Microlay, Miicraft, Rapid Shape, Straumann, W2P
-
Curing Devices: Otoflash, Dentalfarm
-
-
Easy to polish
-
Highest bending & breaking strength
-
High accuracy of fit
-
MMA- and THF-MA-free, tasteless
Clear-transparent formulation for visual control in the working area. Process reliability through high initial hardness and final strength.
Low-viscosity approach for low material consumption and more rapid cleaning. Highest mechanical bending and breaking strength, without being brittle.
Neutral odour and taste.
TGA Registered Medical device Classs IIa
1) Dentistry: Orthodontic resins (in keeping with the standard at room temperature)
2) Biological assessment of medical devices – Part 1: Assessment and testing in the context of a risk management system
|
Parameters |
Standard |
Unit |
Results |
|
Flexural strength Flexural modulus |
DIN EN ISO 20795-21) DIN EN ISO 20795-21) |
MPa MPa |
> 80 > 2000 |
|
Water absorption Solubility Hardness |
DIN EN ISO 20795-21) DIN EN ISO 20795-21) – |
μg/mm³ μg/mm³ Shore D |
< 32 < 5 > 82 |
|
Biocompatibility |
DIN EN ISO 10993-12) |
– |
fulfilled |
Related Products
Detax Freeprint SPLINTMASTER FLEX 385 DLP 3D Printing Resin 04432 - 1000g
3D Print Resin Stock Holding Information and Leadtimes: Initally given the nature of the digital la..
NK Optik Otoflash G171-6 with N2 Nitrogen GAS PORT Light Curing Unit - Stroboscopic-Light - 1 Unit
NK Optik Otoflash G171-6 with N2 GAS PORT - 1 Unit With Nitrogen attachment for CE requirements..
Detax Freeprint MODEL 2.0 385 DLP 3D Printing Resin – Caramel – 1000g
3D Print Resin Stock Holding Information and Leadtimes: Initally given the nature of the digital la..